Common engineering solids usually have coefficients of thermal expansion that do not vary significantly over the range of temperatures where they are designed to be used, so where extremely high accuracy is not required, calculations can be based on a constant, average, value of the coefficient of expansion.
To more accurately calculate thermal expansion of a substance an equation of state must be used, which will then predict the values of the thermal expansion at all the required temperatures and pressures, along with many other state functions.
For solid materials with a significant length, like rods or cables, an estimate of the amount of thermal expansion can be described by the
 ratio of strain:
ratio of strain: is the length before the change of temperature and
is the length before the change of temperature and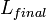 is the length after the change of temperature.
is the length after the change of temperature.For most solids, thermal expansion relates directly with temperature:
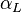 is the linear coefficient of thermal expansion in inverse kelvins.
is the linear coefficient of thermal expansion in inverse kelvins.Materials with anisotropic structures, such as crystals and many composites, will generally have different linear expansion coefficients
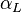 in different directions. Isotropic materials, will by definition have the same
in different directions. Isotropic materials, will by definition have the same 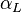 in every direction. For an isotropic material,
in every direction. For an isotropic material, .[1]
.[1]
Thermal expansion generally decreases with increasing bond energy, which also has an effect on the hardness of solids, so, harder materials are more likely to have lower thermal expansion. In general, liquids expand slightly more than solids.
Absorption or desorption of water (or other solvents) can change the size of many common materials; many organic materials change size much more due to this effect than they do to thermal expansion. Common plastics exposed to water can, in the long term, expand many percent.
For an ideal gas, the volumetric thermal expansivity (i.e. relative change in volume due to temperature change) depends on the type of process in which temperature is change. Two known cases are isobaric change, where pressure is held constant, and adiabatic change, where no work is done and no change in entropy occurs. In an isobaric process, the volumetric thermal expansivity, which we denote βP, is:
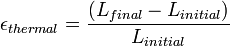
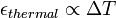
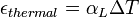
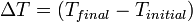



Tidak ada komentar:
Posting Komentar